<= replace with profile picture

| Quote: put text here |
|
put text here
Baby we light up your world like nobody else!!!!!!!!!!
|
| Status update:at least 3 updates |
|
at least 3 updates
4th of July was crazy! Exploded all over the place.
Had to get oil put on me again so I don't randomly react.. Ugh. I'm just too reactive.. :(
My name comes from rubidus which means "the deepest red".
|
| Overview: |
|
3-5 sentences about the elements. (physical and chemical properties)
I am a soft silvery metal in the alkali metal group.
I am the second most reactive metal.
I react spontaneously in air and water.
When I react with water, it releases hydrogen gas, which catches fire.
|
| Information: |
|
|
Relationship Status: single
|
|
|
Parent: Robert Bunsen and Gustav Kirchhoff
|
|
|
network: metal
|
|
|
valence electrons: 1 valence
|
|
| reactivity: react violently with water and 2nd most reactive element. Cesium is more reactive. |
|
| state of matter: solid at room temperature |
|
| Location on Periodic Table: group 1 period 5 |
|
|
Birthday: 1861
|
|
|
Hometown: Heidelberg, Germany
|
|
|
Group: group 1
|
|
| Activities & Interests: |
|
2-3 sentences about the elements. (physical and chemical properties)
Physical properties: While being a metal, rubidium is soft and silvery. Rubidium is an alkali metal. Rubidium is the second most reactive metal, cesium being the first. It can't be existent just anywhere because it is EXTREMELY reactive.
|
| Places: |
|
"check in" with at least three places the element can be found
|
|
Checking in at: Hanging with lepidolite. MY ORE!
Checking in at: Seawater! Loving all the fish :)
Checking in at: Chilling with my buds at the mineral springs. B-)
|
| Photo: |
|
Create a photo album:
- every photo must have a caption
- tag other elements in the pictures
|
|
|
My Friends and I around the World
|
|
|
 Me and water reacting. SMOKE! Me and water reacting. SMOKE!  Had to get stored in hydrogen this time.. Had to get stored in hydrogen this time..
 Potassium and I. Great show! Potassium and I. Great show!
|
|
|
|
|
|
Electron Shell Diagram
P+ =37
N= 48
E= 37
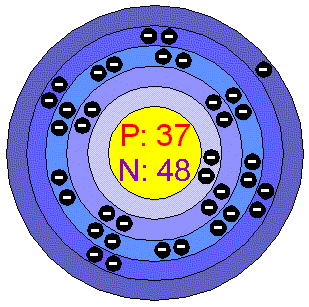
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
list the pages you are a "fan" of here
Alkali Metals Group Page
Rubidium
Back: Periodic Network Project
Comments (5)
Anne Alarcon said
at 2:22 pm on Oct 16, 2012
Why are you so reactive?
Who is more reactive than you?
Rubidium said
at 6:13 pm on Oct 30, 2012
Rubidium has very low ionization energy so they give up their outer electron very easily. Cesium is more reactive than rubidium.
Mrs. Lockwood said
at 8:26 pm on Oct 23, 2012
Rubidium can react violently with other elements and compounds. How is rubidium stored?
Rubidium said
at 6:13 pm on Oct 30, 2012
Rubidium is stored under oil so it won't react.
Beryllium said
at 8:35 pm on Nov 7, 2012
I hear you're violently reacting to water.
You don't have permission to comment on this page.